29 May 2025
The BCG is one of the oldest vaccines ever developed against one of the oldest diseases plaguing humanity. Yet, despite being more than a century old, the vaccine is brimming with untapped potential that holds far-reaching implications in the fight against infectious diseases and cancer.
BCG in New Zealand: Out of sight, out of mind.
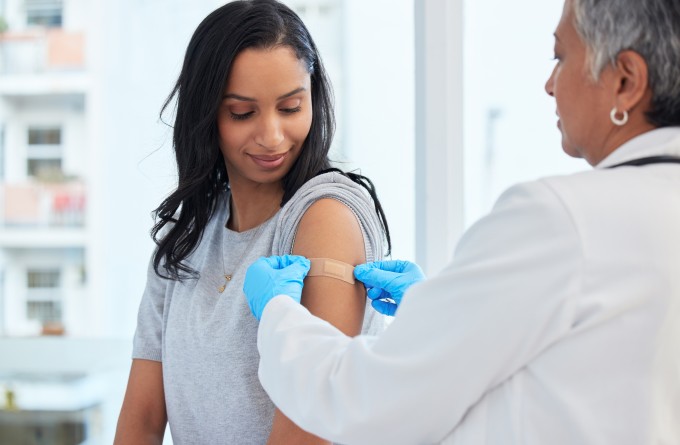
Many Kiwis may recognise the telltale sign of the BCG vaccine. Depending on age and birthplace, perhaps on their own arm, a parent's, a grandparent's or even a colleague's. The small scar on the upper arm, in the shape of a hollow, indented circle is a lasting mark of the only WHO-endorsed vaccine against tuberculosis (TB).
The BCG vaccine, combined with the development of several specialised antibiotics from 1940 to 1966, marked a significant step change in humanity’s long, arduous battle against this insidious and formidable disease.
Before this, TB had overwhelmed health systems globally, filling entire hospital wings and requiring dedicated treatment centres. But within just a few short years, it had virtually disappeared as a major cause of death in high-income countries.
Since the 1960s, New Zealand and many other high-income countries have gradually phased out the BCG vaccine from national immunisation programmes as TB rates have declined.
The BCG scar seems like the remnant of a distant, archaic disease that occasionally rears its ugly head as an invisible villain in a period drama or literary classic, causing characters to waste away and succumb to untimely and tragically inevitable deaths.
“Though fading from public memory in New Zealand, the BCG vaccine is still the most widely administered vaccine in the world and for good reason,” says Dr Kerry Hilligan, infectious disease researcher and Team Leader in the Ronchese Laboratory at the Malaghan Institute of Medical Research.
TB is far from a remnant of a distant past. It is a destructive force in many parts of the world. In fact, an estimated one quarter of the world’s population are currently infected with the bacteria that causes TB, Mycobacterium tuberculosis.
“Most of these infected people have no idea they are infected. They are latently infected which means the bacteria lie dormant,” says Dr Hilligan.
A tenth of those infected with the TB bacteria go on to develop active TB, a slow-progressing, debilitating disease that consumes the victim from inside out, earning the disease the terrifying name ‘consumption’. In the last 200 years, TB has taken more than 1 billion lives globally.
These staggering numbers make TB the infectious disease that still causes the most deaths in the world, briefly overtaken by Covid-19 during the recent pandemic.
Most of these cases are concentrated in Asia, Africa and South America. Here, TB patients still fill entire hospital wings and require dedicated treatment centres.
This is because the progress we made in the 20thcentury was not distributed equally. Access to life-saving treatments was uneven, and many lower-income countries did not experience the same dramatic drop in TB rates.

Now, TB is re-emerging as a serious global threat. The bacteria are evolving to resist existing treatments, a condition known as multidrug-resistant TB, which is far more difficult and expensive to treat. To make matters worse, the BCG vaccine, now over 100 years old, offers little protection from TB beyond early childhood. As this disease continues to cause a public health crisis, researchers are re-examining BCG with fresh eyes.
“The BCG vaccine has long been something of an enigma. Originally developed to protect against tuberculosis, it provides only limited protection. Yet over the years, studies have revealed a surprising pattern: populations who receive the vaccine can be protected against more than just TB,” says Dr Hilligan.
“In some cases, vaccinated populations are less likely to suffer from severe respiratory infections and sepsis. These ‘off-target’ effects have long intrigued scientists and have even led to the development of BCG as the gold-standard treatment for invasive bladder cancer.”
“What if understanding the broad immune effects of BCG could help us solve some of the biggest questions in immunology? And what if that knowledge led to more effective vaccines against tuberculosis, broader protection against infectious diseases and even new treatments for cancer?”
These are key questions driving Dr Hilligan. In a recent review published in the Journal of Experimental Medicine, working with her former postdoctoral supervisor Dr Alan Sher, from the National Institute of Allergy and Infectious Diseases in the US, Dr Hilligan shines a light on the untapped potential this century-old vaccine holds. Their work traces how BCG activates multiple arms of the immune system and why fine-tuning these responses could open new doors from antiviral development, cancer treatment and to a more effective tuberculosis vaccine, something that is urgently needed to protect millions of people around the world.
What is BCG?
The BCG vaccine has a fascinating origin story involving cows and potatoes. It was developed in 1921 by French scientists, who were trying to create a vaccine by weakening the bacteria that causes tuberculosis in cows. Over 13 years, they repeatedly grew the bacteria in a mixture containing potato slices soaked in ox bile. This slow and careful process gradually weakened the bacteria so that it could not cause disease but was still strong enough to stimulate the immune system. Calmette and Guérin immortalised their hard work, naming the vaccine after themselves: Bacillus Calmette-Guérin (BCG).
“This type of vaccine is known as a ‘live attenuated’ vaccine, which means it uses a live microbe that has been tamed so the body can learn to fight it without becoming ill,” says Dr Hilligan.
Route of administration
“One of the most striking findings from recent studies is that the way BCG is delivered makes a big difference,” says Dr Hilligan.
Currently, BCG is injected intradermally, just below the skin. In many countries, this is done just hours after birth to give children early protection. This method is safe, convenient and easy to administer, but its effectiveness is limited.
“Recent animal studies have found that by administering BCG intravenously, that is directly into the bloodstream, sterilising immunity can be achieved. This is unheard of with TB!” says Dr Hilligan.
Sterilising immunity is the highest level of vaccine protection. It means the immune system not only stops the disease from causing symptoms but prevents the bacteria from taking hold in the body at all. In these animal studies, BCG delivered through the bloodstream stopped the TB bacteria completely. There was no infection, no replication and no trace of disease.
“Current intradermal BCG vaccines only provide up to ~40 percent protection from TB in children under 5, so this is a huge leap,” Dr Hilligan explains.
Researchers are now ready to ask a bold question: can this remarkable result be repeated in people? If it can, it would represent one of the most important steps forward in TB prevention in more than a century.
“Intravenous delivery of BCG may be more effective because it gives the vaccine access to key parts of the body that intradermal injections cannot reach. When BCG enters the bloodstream, it travels to important immune organs like the lungs, bone marrow and spleen. There, it helps reprogram stem cells to produce more responsive immune cells, especially in the lungs where TB usually strikes.”
Understanding the whole lung: Current research
“These recent findings about the BCG tell us we need to expand our approach to studying the immune system beyond just individual cells. The immune system is a vast network composed of cells that interact with each other and every type of tissue in the body,” says Dr Hilligan.
Dr Hilligan’s current research at the Malaghan Institute is investigating how the lung as a whole responds to infectious disease.
“I’m looking into the contextual information around how our body responds to infections in the lung. Whether that is from specific viruses, bacteria or fungi, what are the commonalities in our immune response? Could we use these common responses to help protect us from future threats?”
This shift in perspective from using BCG narrowly as a TB vaccine to exploring it as an immune training tool could transform how we think about vaccines and immune health more broadly.
Understanding and improving BCG offers us the opportunity to address some of the greatest health threats we face today. By harnessing BCG’s broad immune effects, we may unlock new tools to fight cancer and develop broad spectrum antivirals that strengthen our preparedness for future pandemics.
But perhaps most powerfully, developing a more effective TB vaccine gives us the chance to right the historic injustices of unequal access to life-saving treatments. It is an opportunity to do better, to share protection more fairly, to reach those most in need and to bring humanity closer to finally vanquishing one of its oldest and most devastating diseases.
------------------------------------------------------------------
Listen to Dr Kerry Hillman on Our Changing World: New insights from an old vaccine, 24 June 2025
Related articles

Momentum is everything: advancing CAR T-cell research for future trials and treatments
25 February 2026
NZ-UK research deepens understanding of germinal centres for better vaccine design
27 January 2026

How a quirk of the immune system may play a big role in protecting us from disease
18 December 2025

The nose knows: new research explores next generation of nasal vaccines
2 December 2025

Tracking the journey of the shapeshifting bacteria behind stomach cancer
19 November 2025

Marsden funding to drive discovery and innovation in cancer, allergy and infectious disease research
5 November 2025
