14 September 2023
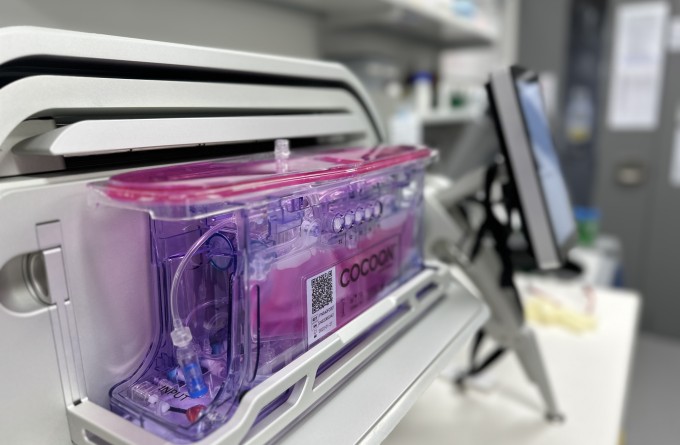
Behind the Malaghan Institute’s recent shift to automated production of CAR T-cells for its ENABLE clinical trial programme is a hi-tech machine called the Lonza Cocoon. Working with it to enable it to safely and successfully transform a patient’s blood cells into effective cancer killers is Research Officer Felix O’Hagan. His work optimising this technology has made him one of the few experts in the world of this new and revolutionary technology.
As the Malaghan Institute's CAR T-cell trial for B-cell non-Hodgkin lymphoma progresses towards phase 2, the need for efficient and scalable CAR T-cell production has never been more critical. The automation of CAR T-cell manufacture has the potential to not only expand the trial's reach to more patients, but could position CAR T-cell therapy as a viable and accessible treatment option for lymphoma patients nationwide if the trial is successful.
The Lonza Cocoon system represents a major advancement in CAR T-cell production. Felix has been working with the Cocoon since late 2021, optimising its automated process to produce consistent and safe CAR T-cell products that can be used for clinical trials.
“The highlight of my work on the Cocoon has been that it has such an immediate impact on people.”
“The process of producing CAR T-cells in the Cocoon is actually a relatively simple process, as many of the laborious manual steps have been automated,” says Felix.
First, blood cells from the patient undergo a selection process to obtain a pure population of T-cells. Once enough T-cells are present, a gene sequence that provides instructions to make the chimeric antigen receptor (CAR) is inserted. The T-cells can then produce the CAR protein on their surface, becoming CAR T-cells. These CAR T-cells have the potential to target and eliminate the cancerous lymphoma cells in the patient’s body.
“The resulting CAR T-cells undergo rigorous quality control testing to determine if a sufficient number of T-cells have successfully transformed into CART-cells, and that these cells are safe to give to the patient,” says Felix.
“The Cocoon streamlines the entire production process, making it more efficient and cost-effective compared to the manual production of CAR T-cells. Now that we know it works reliably, it will be a game-changer on many different levels.”
CAR T-cell production is a labour-intensive process when done manually. It takes the skilled GMP technicians at the institute approximately 40 hours of operator time per patient over two weeks.
“The Cocoon reduces operator time per CAR T-cell product by at least half. Future manufacturing improvements are likely to reduce this even further,” says Felix.
“This means more patients can receive CAR T-cell therapy in a shorter timeframe.”
This significant reduction in the operator hours required for each CAR T-cell production means that members of our highly-skilled GMP team can be reallocated to the next patient, or other critical aspects of therapy development such as research and quality control.
“Where manually we can only manufacture one patient’s CAR T-cells at a time to mitigate the risk of cross-contamination, the Cocoon is a closed system that allows for the simultaneous production of multiple patients' cells in one room. Again, this increases the overall production capacity for CAR T-cells,” says Felix.
“Producing CAR T-cells in a closed, automated system means tasks can also be performed remotely with minimal human interaction, reducing the risk of contamination and ensuring product integrity.”
“The product of the Cocoon is being used to treat actual cancer patients and that is a truly motivating thing.”
Felix, a Wellingtonian who attended Wellington High School, is a self-described “know-it-all”.
“I have a compulsive need to know all the details of how something works whether that was in high school biology or now with my work on the Cocoon.”
He went to the University of Otago to do his undergraduate studies in biomedical science.
“I ended up doing my Honours project in gastrointestinal organoids, the last thing I thought I’d be working on. That’s when I realised anything in science could be interesting, it’s the process that’s the real captivating factor.”.
Felix came to work at the Malaghan Institute in mid-2021.
“The highlight of my work on the Cocoon has been that it has such an immediate impact on people. The product of the Cocoon is being used to treat actual cancer patients and that is a truly motivating thing,” he says.
“It’s so satisfying to get updates on how much of a difference the CAR T-cells are making to patients’ lives. It really brings the research to life.”
Related articles

Phase 2 clinical trial underway to confirm effectiveness and safety of NZ’s first CAR T-cell cancer therapy
23 July 2024

RNZ Our Changing World: Targeting bacteria, and health inequities
4 July 2024

New clinical research aims to reduce stomach cancer rates and disparities in New Zealand
28 May 2024

A revolution in cancer care is near; here’s what’s needed to make it happen
9 May 2024
Significant milestone reached in first NZ CAR T-cell trial as preparations made for larger phase 2 registration trial
25 March 2024

RNZ: Engineering immune cells to kill cancer
5 November 2023
