A revolutionary approach to fighting cancer, CAR T-cell therapy has the potential to transform cancer treatment. We're working to make it accessible and affordable for all New Zealanders in need.
CAR T-cell therapy stands for Chimeric Antigen Receptor T-cell therapy. A one-off treatment, it works by redirecting a patient’s own immune cells (T-cells) in the laboratory, to directly identify and attack cancer cells. These modified T-cells are then returned to the patient where they can attack and destroy cancer cells. CAR T-cells have the potential to act as ‘living drugs’, providing long-term protection against relapse.
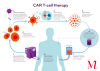
CAR T-cell therapy has the potential to target a range of cancers, with research and clinical trials underway worldwide. However, to date, it has been effective for treating certain blood cancers, including certain lymphomas, B-cell leukaemias, and myeloma. In Australia, the USA and parts of Europe and Asia, CAR T-cell therapies for these types of cancer are licensed for routine use.
In New Zealand, the Malaghan Institute’s CAR T-cell programme includes the country’s first CAR T-cell trial, with on-shore CAR T-cell manufacture and delivery. Our goals are to conduct cutting-edge trials of new CAR T-cell therapies, and to leverage our nationally-unique manufacturing, regulatory and clinical experience to help introduce CAR T-cell therapies as a standard of care in New Zealand.
CAR T-cell Clinical Trial Programme
The Malaghan Institute, in partnership with Wellington Zhaotai Therapies is developing and trialling a new CAR T-cell therapy in New Zealand for patients with certain types of lymphoma. The treatment we are working on is a ‘third generation’ CAR T-cell therapy, which we hope may be at least as safe and effective, and easier to deliver, than treatments currently available elsewhere.
ENABLE-2 phase 2 clinical trial
In July 2024, a phase 2 clinical trial, ENABLE-2, got underway on the back of promising phase 1 results.
The goal of ENABLE-2 is to confirm the effectiveness and safety of our CAR T-cell therapy and, ultimately, to get it registered for routine use in New Zealand and Australia.
Within ENABLE-2, 60 adults with certain types of relapsed or refractory large B-cell non-Hodgkin lymphoma will be treated over two years. The trial is taking place at Wellington, Auckland City and Christchurch hospitals, with automated manufacturing and delivery of CAR T-cells by our partners BioOra Limited, at the Malaghan Institute in Wellington.
After assessing the dose and safety of this new CAR T-cell therapy in the ENABLE phase 1 trial where patients had exhausted all other treatments, patients will be treated earlier in their treatment pathway for ENABLE-2. We are hopeful that treating patients earlier – as a second- or third-line therapy – will result in even better CAR T-cell outcomes as their immune system function may have been less damaged by their prior cancer treatments.
The safety profile of our new CAR T-cell therapy also means we can deliver it as an outpatient treatment, lowering the burden on patients and their whānau, and reducing costs to the health system.
The main barriers to CAR T-cell therapy globally are the burden of managing side effects and the cost of the CAR T-cells themselves. By combining an improved safety profile with cost-effective manufacturing, we aim to address both issues.
Results of ENABLE phase 1 clinical trial
Our ENABLE phase 1 safety trial got underway at Wellington hospital in late 2019 to determine the optimal dose of our novel CAR T-cell therapy. The trial treated participants across the country with certain types of relapsed and refractory B-cell non-Hodgkin lymphoma, who had exhausted other treatment options.
Preliminary results of the first 21 patients treated in the trial’s dose escalation cohort, presented at the American Society of Hematology meeting in December 2023, found no limiting toxicities at any of the doses tested. Importantly, none of the participants developed neurotoxicity or severe cytokine release syndrome – common side effects of some commercial CAR T-cell therapies. The trial also showed promising effectiveness, with around half of the participants’ lymphomas in complete response three months after receiving the treatment – that is, no signs of cancer in the body.
These results suggest our new CAR T-cell therapy may reduce risk of severe side effects, while remaining effective.
A further nine patients were treated at the optimal dose as part of a dose expansion cohort, with outpatient management and automated CAR T-cell manufacturing by our partners BioOra using Cocoon technology. Phase 1 trial enrolment and treatment is now complete. Trial participants remain under follow-up, with a primary analysis of all 30 patients treated conducted in late 2024 and presented at the American Society of Hematology meeting in December. Preparation of results for publication is underway.
Trial recruitment
The Malaghan Institute is not a provider of health services and does not recruit patients to clinical trials directly. Patients should speak with their haematologist or oncologist about whether this, or other conventional or clinical trials, might be an option for them. Referrals must be made by a relevant specialist to a trial investigator.
A public summary of the ENABLE-2 trial is available on the ClinicalTrials.gov website.
CAR T-cell research programme
In parallel with our clinical trial programme, our CAR T-cell research programme is focusing on improving the safety and effectiveness of current CAR T-cells, and expanding this cutting-edge technology to treat a wider range of cancers, including solid tumours.
Support bringing CAR T-cell therapy to Aotearoa New Zealand
Join us in making CAR T-cell therapy accessible and affordable for all New Zealanders in need.
Find out more about how you can help make life-saving treatments like CAR T-cell therapy available in New Zealand, or contact the Malaghan Institute's philanthropy specialist An-Li Theron.
Or, if you're keen to support our efforts in other ways, contact the Malaghan Institute's communications team.
ASSOCIATED RESEARCH GROUPS
Related News

Malaghan CAR T-cell cancer therapy trial expands to Christchurch and Auckland
23 June 2025

Kjesten Wiig: bringing life-changing treatments to life
27 February 2025

Cancer Research Trust grant to improve CAR T-cell therapy
12 February 2025

Community partnerships: Zephyr Consulting empowering emerging scientific talent
18 November 2024

CAR T-cell therapy, the battle of the blood cells
26 September 2024

Phase 2 clinical trial underway to confirm effectiveness and safety of NZ’s first CAR T-cell cancer therapy
23 July 2024
