23 June 2020
A team at the Malaghan Institute has made a significant finding investigating why glioblastoma cells are resistant to treatment – one that could lead to new treatment options for patients suffering from this aggressive brain cancer.
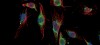
Glioblastoma cells under the microscope
Glioblastoma patients have poor survival rates, partly due to the cancer’s unique ability to ‘bounce back’ from treatment. Malaghan Institute Research Associate Dr Melanie McConnell and her team believe this survivability may come down to a single molecule – BCL6.
BCL6 is a molecule normally expressed by immune cells that makes up an important part of the adaptive immune response. In order for immune cells to make the full spectrum of antibodies and antigen receptors needed to do their job, sections of the immune cell’s DNA are ‘chopped up’ and shuffled into hundreds of unique combinations. Normally, this type of DNA alteration or damage is a sign that something is wrong inside the cell, and such cells are marked for destruction. To avoid this, BCL6 plays a key role, acting as a ‘hall pass’, preventing the immune cell from being destroyed and allowing it to carry out its vital role. However, for glioblastoma patients, BCL6 is turning up in very unexpected places.
“For some reason, we’re seeing this molecule that’s normally expressed in immune cells, turning up in brain cancer. It should never be there,” says Dr McConnell, whose paper ‘The oncogene BCL6 is up-regulated in glioblastoma in response to DNA damage, and drives survival after therapy,’ was recently published in PLOS ONE. The paper was also named paper of the week, ahead of around 600 papers across all fields of science the journal covers.
“What we’re seeing, in the vast majority of our samples of glioblastoma patients, is the presence of BCL6. These patient’s tumours are highly resistant to treatment – chemotherapy, radiation, you name it. BCL6 acts like an invulnerability cloak, letting the tumour grow even while we’re throwing everything at it.
“However, what we’ve also shown in our preclinical models, is that when we knock out BCL6, these cells fall over almost instantly from therapy. While these may just be cells in a petri dish, the correlations are very clear that BCL6 is playing a critical role in cell survival.”
Chemotherapy and radiation therapy work by directly acting on the DNA of cancer cells, damaging it and leaving the cells incapacitated. However, in the presence of BCL6, this otherwise lethal DNA damage isn’t registered as such, allowing the cells to continue growing and dividing unimpeded.
While BCL6 is necessary for a functioning immune system, having it in brain cancer cells is troubling. Dr McConnell and her team are investigating how this molecule ends up being expressed, as well as looking at possible therapies that block its function in the brain.
“There are a couple of emerging drugs being trialled in the States that block the function of BCL6 which look really promising,” says Dr McConnell. “However, these have been developed for treating blood cancer, so we don’t know yet if they’d translate to brain cancer. We don’t know if all these drugs can cross the blood-brain barrier. We also don’t fully understand if the BCL6 expressed by glioblastoma looks and acts in quite the same way as the BCL6 expressed by the immune system. That’s the focus of our research moving forward, to get a better understanding of BCL6 in the brain, how it’s being expressed and whether stopping it is really enough to knock out tumours in glioblastoma patients.”

Above: Glioblastoma cells before (left) and after radiation treatment. Prior to treatment much of the BCL6 (green) is found in the cytoplasm, with some protein in the nucleus. The effects of radiation produces more BCL6 and causes it to migrate into the nucleus where it binds to the genome and changes gene expression to ensure the cell ignores the damage done by the radiation.
This research is funded by the Cancer Society of New Zealand, the Cancer Research Trust NZ and Victoria University of Wellington.
Related news

Malaghan CAR T-cell cancer therapy trial expands to Christchurch and Auckland
23 June 2025

Horizontal mitochondria transfer: 10 years on from a groundbreaking discovery
7 May 2025

Kjesten Wiig: bringing life-changing treatments to life
27 February 2025

Cancer Research Trust grant to improve CAR T-cell therapy
12 February 2025

World-renowned cancer pathologist joins the Malaghan Institute as Distinguished Research Fellow
19 December 2024

New Zealand to New York and back again: Malaghan researcher tackling liver cancer
18 November 2024
