7 May 2025
Considering the extent to which the human body is studied, it’s rare these days to find a truly unique biological process. Yet, that’s exactly what Malaghan Professor Mike Berridge and his colleagues did in 2015, turning an observation on how cancer could bounce back after treatment into a brand new field of biology and cancer research.
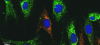
A 2013 image showing mitochondrial transfer from mouse bone marrow stromal cells (green) bone marrow stromal cells with red stained mitochondria.
Now, 10 years on from discovering what is now known as horizontal mitochondrial transfer (HMT), what have we learned, and what clinical implications does it have in fighting disease? Prof Berridge recently published a commentary on the state of HMT and where their field is headed.
Mitochondria wanted – please donate
Mitochondria are like the currency of the cellular world. They’re found in almost every kind of cell, tiny structures whose main role is to facilitate the production of energy. Mitochondria provide chemical energy to a cell in a way similar to a battery does. Cells rich in healthy mitochondria flourish, while those depleted or with defective mitochondria wither.
Prior to this discovery, it was thought mitochondria were proprietary to the cell that made them. In other words, mitochondria never strayed outside of their home. We now know that’s not the case.
However, the extent to which mitochondria can be bartered, traded and – in some cases – outright stolen is something scientists are still coming to terms with, terms which may have significant implications from cardiovascular and neurological disease to cancer treatment.
In 2015, Professor Berridge and his colleagues at the Institute of Biotechnology in the Czech Academy of Sciences were first in the world to confirm mitochondria could be transferred between living cells – horizontal mitochondrial transfer. ‘Horizontal’ refers to how, unlike a parent cell donating its mitochondria to its daughters as it divides, mitochondria are being passed across from one cell to another through tiny nanotubes or extracellular vesicles. A cellular handshake, if you will.
The collaboration showed that if you mix healthy donor cells (with mitochondria) with cancer cells whose mitochondria have been damaged and rendered useless, mitochondria from the healthy cells will eventually make their way to the cancer cells in the body, and those cancer cells will start growing again.
“Prior to this the central dogma was that cancer cells utilised rapid but less efficient methods of energy production as their primary means to survive treatment. It wasn’t until we were able to prove that mitochondrial DNA from healthy donor cells were turning up in these depleted cancer cells that put to bed this line of thinking,” says Prof Berridge.
Published in Cell Metabolism, the research opened the door to one of the many strategies cancers can use to shrug off treatments designed to kill them. Like a wolf in sheep’s clothing, a damaged cancer cell can send distress signals to neighbouring cells, and like any good neighbour these unwitting cells donate what they can – ultimately helping the cancer cell get back on its feet.
Cancer: mitochondria predator
Consider this. You’re a newly-minted T-cell, fresh-faced, conducting your first patrol of the body. Out of nowhere you’re mugged by a cancer cell. You don’t get a good look at its face before it disappears into the crowd of other cells, but you’ve already been robbed of your mitochondria, your precious energy reserves.
What’s worse, the cancer cell has given you its dodgy, damaged mitochondria, and so you no longer have the energy to mount a counter-attack.
“Cancer cells can get rid of their damaged mitochondria to immune cells resulting in immunosuppression and pick up, or steal, healthy mitochondria from T-cells, macrophages and stromal cells, leading to tumour proliferation,” explains Prof Berridge.
This isn’t an isolated phenomena, limited to a few special cases. A decade on from the original findings, HMT has been popping up again and again, implicated or contributing to the proliferation of many different cancers.
“HMT has now been shown in more than 20 cancers including lung, breast, ovarian, prostate, colon, skin and liver, as well as leukaemias and multiple myeloma. Chemotherapy and radiotherapy have been shown to promote HMT in leukaemias and glioblastoma, enhancing the fitness of the cancer cells, increasing therapeutic resistance.”
However, while HMT may be making cancer cells harder to kill, it may also be the answer to unlocking powerful new therapies.
A mito-powered future
In his commentary, Prof Berridge writes about how controlling the traffic of mitochondria between cells could dramatically improve how we treat disease.
“In general, HMT promotes resistance to therapy. Pharmacological processes that interfere with HMT to and from cancer cells have the potential to halt tumour progression and improve the immune system’s antitumour response,” says Prof Berridge.
“However, in other areas, say for CAR T-cell therapy, mitochondria from healthy stromal cells could be passed to CAR T-cells to improve their metabolic fitness and counter T-cell exhaustion – a current limitation of this therapy.
“What’s more, in cardiovascular diseases and in tissue repair, HMT has been shown to have beneficial effects.”
While global research is still a long way off utilising HMT in a therapeutic setting, this emerging field of biology is already gaining significant momentum.
“This area of research is very new,” says Prof Berridge. “As research continues to uncover more about the role of HMT, the specific characteristics of mitochondria being transferred and the effects on recipient cells will pave the way for strategies to disrupt HMT and potentially inhibit tumour growth and lower resistance to therapy.”
Related articles

Tracking the journey of the shapeshifting bacteria behind stomach cancer
19 November 2025

Marsden funding to drive discovery and innovation in cancer, allergy and infectious disease research
5 November 2025

Eradicating H. Pylori bacterial infection to reduce stomach cancer
30 October 2025

Developing next generation CAR T-cell therapies for more equitable cancer care
30 October 2025

Making local impact using global training in liver cancer research
30 October 2025
Faster CARs: overcoming cellular exhaustion to enhance cancer immunotherapies
29 October 2025
