9 November 2022
Kirsty Wakelin and her team are the people who manufacture CAR T-cells from the cells of cancer patients on the Malaghan Institute’s CAR T-cell trial. She takes us through the meticulous process required to provide the highest quality CAR T-cell treatment to patients.
As a patient receiving CAR T-cell therapy on the Malaghan’s ENABLE clinical trial, the process can seem very simple. Patients go into hospital to have their white blood cells extracted. They go home and come back in four weeks to receive a small, 2ml injection containing the modified version of their extracted T-cells, a specialised type of white blood cell, now CAR T-cells. The injection itself is over in under two minutes. The process of taking the patient’s cells and modifying them so they can target cancer cells can seem like magic. However, there is a specialised team at the Malaghan Institute working behind the scenes to ensure that patients receive functioning, high-quality CAR T-cells that target their cancer. Kirsty Wakelin is Senior Production Technician and part of the team responsible for the journey the extracted T-cells make to transform into CAR T-cells, ready to be injected into patients for treatment.
“It’s rewarding to see what we’re doing in the lab being used to treat patients for cancer. These patients would have otherwise not have access to this treatment so I understand the significance of getting the process right,” says Kirsty.
T-cells are naturally programmed to circulate around the body and detect specific signals on other cells which show they are defective or have been hijacked by a virus. On detecting these cells, the T-cell receives two signals: first to release its internal safety switch, called the co-stimulatory domains, which help prevent the T-cell from killing an otherwise healthy cell; and the second signal which activates its kill function to destroy the defective cell.
“I look forward to when CAR T-cell therapy can be administered to people as a standard of care rather than a last resort."
T-cells are naturally programmed to circulate around the body and detect specific signals on other cells which show they are defective or have been hijacked by a virus. On detecting these cells, the T-cell receives two signals: first to release its internal safety switch, called the co-stimulatory domains, which help prevent the T-cell from killing an otherwise healthy cell; and the second signal which activates its kill function to destroy the defective cell.
When making CAR T-cells, T-cells are extracted from cancer patients and genetically modified. Kirsty and her team have the important role of equipping these T-cells with the ability to target B-cell lymphoma, the specific type of blood cancer these patients have.
“Once we receive the T-cells from the patient, we use lentiviral vectors to add a gene that allows the CAR T-cell to recognise the cancer cells and destroy them,” says Kirsty. The lentiviral vectors act as vehicles that deposit these new genes into the DNA of the T-cells at the centre of the cell.
“The whole process from receiving the patient’s T-cells to providing the CAR T-cells that are ready to be injected into the patient is very meticulous. It requires us to take several precautions to ensure that the cells are functional and aren’t contaminated.”
Kirsty and her team only work on producing one patient’s CAR T-cells at a time to prevent contamination with other cells.
“Genetically modifying the T-cells takes two weeks. Over the following week, we run several tests to ensure the quality of CAR T-cells,” says Kirsty.
“The number and location of the CAR gene inserted into each cell varies. We test that enough of the T-cells have received the genes to make them into CAR T-cells. We then test these new CAR T-cells against cancer cells in the lab to ensure they are effective.”
Kirsty’s team package these CAR T-cells into a small 2ml vial, ready to be administered to patients. If the treatment is successful, the CAR T-cells will go and hunt down the cancer cells in the patient’s body and destroy them.
“I feel grateful to be given the chance to help the patients on this trial and thankful that they are also helping us to keep developing and improving our treatments.”
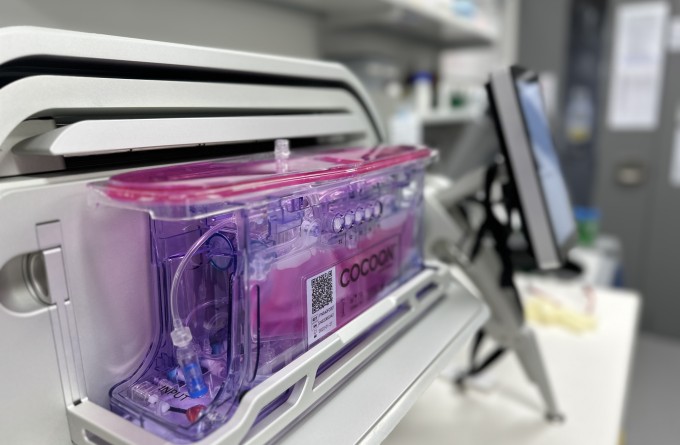
“The road forward from here is about getting the manufacturing process to be more efficient while maintaining the highest quality. We’re hoping we can implement the Cocoons into our process in the near future.”
The Cocoons are machines that will automate the process, allowing each patient’s cells to be modified and processed in a closed environment. This will allow more than one patient’s cells to be processed at one time while ensuring no contamination occurs. The Cocoons will be especially important if the clinical trial is successful as it will allow the therapy to be scaled up and made widely available across Aotearoa New Zealand.
“I look forward to when CAR T-cell therapy can be administered to people as a standard of care rather than a last resort. Currently, patients are only eligible to be on the trial once they have exhausted other options including chemotherapy which can severely weaken your immune system, including T-cells,” says Kirsty.
“If CAR T-cell therapy was offered as an option before the patient goes through as much chemotherapy, the T-cells we have to work with will be stronger, meaning the resulting CAR T-cells will also be stronger.”
Originally from Whakatāne, Kirsty was always inquisitive in nature.
“I was always that kid who said ‘But why? How? How does that work?’,” says Kirsty.
“I’m a very logically minded person so maths and science just naturally drew me in. I thought I wanted to be a doctor and then a dentist but actually I just didn’t know what careers were available to me at the time.”
She went on to study Biomedical Science at the University of Otago and discovered her love of lab work. For Kirsty, her current role ticks all the boxes.
“CAR T-cell therapy is such a revolution in cancer therapy and I’m thrilled to be a part of it. In addition to the fact that my work might literally be changing someone’s life, I just love perfecting processes, especially in the lab,” says Kirsty.
“I feel grateful to be given the chance to help the patients on this trial and thankful that they are also helping us to keep developing and improving our treatments.”
Related articles
Significant milestone reached in first NZ CAR T-cell trial as preparations made for larger phase 2 registration trial
25 March 2024

Location, location, location: study finds where MAIT cells live may determine their role in allergic disease
12 February 2024

Freemasons New Zealand renews support for CAR T-cell therapy
10 November 2023

RNZ: Engineering immune cells to kill cancer
5 November 2023

Trial results offer hope to Kiwis with incurable blood cancer
3 November 2023

BioOra appoints CAR T-cell therapy pioneer, Professor Carl June to Board
31 October 2023
